We Are All Reservoir Engineers in Some Ways
The oil and gas industry is often seen as an interdisciplinary, complex system in which professionals seem to know a lot about the subsurface they are dealing with and they are able to manage huge reservoirs with high confidence. However, a lot of the same concepts can be used in everyday life too.
People rely on porous materials every day, ranging from the sponges they use for cleaning to the breathable fabrics in their clothing. The main difference between laypeople and reservoir engineers is that while the former deal with porous media according to common sense and experiential knowledge, the latter tries to understand the physics behind each phenomenon. Also, people daily—albeit subconsciously— take advantage of the same processes that have been the basis of the success of the petroleum industry over the years.
In this article, three everyday examples of porous media are shown and explained from the point of view of a reservoir engineer, showing that we are all reservoir engineers in some ways.
A Piece of Bread
Imagine that you have just finished eating pasta with tomatoes and a thick film of sauce creates a bed on the bottom of the plate. Would you consider using a piece of bread to recover the remaining sauce? How about leaving it inside the small pool?

As soon as the bread touches the fluid, you notice that not only is the mixture absorbed by the porous media, but that it also starts to rise throughout the crumb. This is the effect of capillary pressure, the pressure between two immiscible fluids (sauce and air in this case), resulting from the interactions between the fluid and the walls of the bread crumb. Although, there is a combination of capillary rise and absorption by the bread crumb, a crucial role is played by crumb alveolation.

The concept of capillary pressure is related to that of surface tension. If we consider the system shown in Fig. 1, made up of a bubble of oil inside water, the mechanical equilibrium at the red point foresees:

Where FInterface tension is the force at droplet interface and FPressure is the force related to the pressure difference between oil and water and, thus, inside and outside the droplet. Therefore:

Where γ is the interfacial tension, R is droplet radius, Pi is the density of the fluid inside the droplet, Po is the density of the fluid outside the droplet, and θ is the contact angle between them.
Moreover, from Stevin’s law:

Where Pc is the capillary pressure, g is the gravitational acceleration, and h is the droplet depth.
Therefore, just replacing the latter into the force balance, the Young-Laplace equation is obtained:

The importance of this equation lies in its flexibility because it can be applied not only for the calculation of capillary pressure of an oil droplet, but also for the estimation of capillary rise (h in the equation) and, moreover, on the capillary rise of water inside a very small tube.
According to the equation above, the shape of the bread crumb cavities is a key factor influencing the height to which the sauce rises. The thinner the cavities, the higher the height, and the higher the amount of sauce that imbibes bread crumb, the higher the efficiency of the imbibition process in terms of sauce recovery. A reservoir engineer would say that there is a transition zone created by capillary rise, in which both air and sauce are present in the porous medium.
A Cup of Coffee
In 1933, Alfonso Bialetti filed a groundbreaking patent for the moka coffee pot. From that moment, its design underwent few changes due to the high efficiency and simplicity of the process involved. As water is heated up inside the small tank, it starts to form a gas cap.

Gas pushes water inside the conduit, forcing it through the coffee powder. The constant heat supply guarantees continuous vaporization and pressure support, eliminating the need for a pump to allow the coffee to flow out.
Pressure support is a crucial mechanism in the oil and gas industry, since it is fundamental to enable the production of hydrocarbons (HCs). There is a class of reservoir that shows the presence of a gas cap. This cloud of gas that stands upon the oil pool is made of lighter HCs that are no longer soluble in the underlying liquid due to pressure and temperature conditions. In other words, the reservoir pressure is lower than the saturation pressure of the fluid at reservoir temperature. Once production begins, the reservoir starts to be depleted. At the same time, from one side, the gas solution is filling the gas cap. On the other side, the gas cap expands, providing additional energy and pressure to support oil production. Oil recovery due to gas-cap drive can reach 40% (Satter and Iqbal, 2016).
Despite Bialetti not being a reservoir engineer, his moka pot serves as a homemade-scale experiment demonstrating how gas pressure supports the flow of water through the porous coffee grounds, ultimately allowing the coffee to be produced at surface. This mechanism is analagous to the oil displacement resulting from the gas injection process.
Stain Removal
Imagine that part of the tomato sauce mentioned earlier has spilled on your shirt, making a wide stain on it. There is an affinity between the grease inside the sauce and the fabric of your shirt that must be broken. To remove the stain, you put it into the washing machine with detergent and wait for the magic, if it happens.
How does it actually remove the stain? Soap is made of surfactants. Surfactants are made of a hydrophilic head (which is attracted by the water) and some hydrophobic tails (which are attacted by the grease) as seen in Fig. 2.
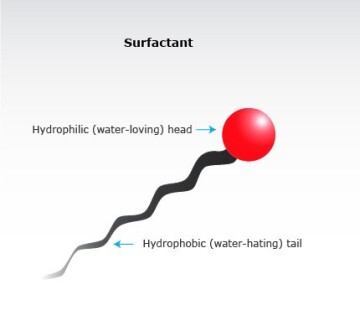
The attractive forces between heads and tails are so strong that grease is removed from the fabric and washed away with water.
Therefore, the basic principle is to make the shirt wetter by water rather than with sauce in order to remove the stain. The limitation is that in the washing machine, other clothes become twisted together, creating a series of caves and canals through which our mixture of water and soap must move, with the hope of contacting the grease stains.
Surfactants are used similarly in the petroleum industry. As one of the most used enhanced oil recovery (EOR) techniques, surfactants injection is utilized because they can act in two main directions according to their own properties (Massarweh and Abushaikha, 2020).
1. Reducing interfacial tension between oil and water. In particular, the capillary number (Nc) is introduced as the ratio between viscous forces and interfacial forces:

Where μw is water viscosity, uw is water velocity, φ is porosity and σow is the interfacial tension between oil and water. The reduction of σow leads to interfacial tension decrease, and, thus, capillary number increment. This results in smaller residual oil saturation.
2. Altering rock wettability (Fig. 3).
The former is fromuseful to increase the sweep efficiency and to reduce oil snap-off due to spontaneous water imbibition, while the latter is mainly exploited in carbonate reservoirs to make them wetter.
While common sense leads us to think we are cleaning our clothes, the reservoir engineer will affirm that they are enhancing grease recovery.
For Further Reading
Fluid Saturation and Capillary Pressure by P. Glover, Petrophysics MSc Course Notes.
Primary Recovery Mechanisms and Recovery Efficiencies by A. Satter, G.M. Iqbal, Reservoir Engineering: The Fundamentals, Simulation, and Management of Conventional and Unconventional Recoveries
The Use of Surfactants in Enhanced Oil Recovery: A Review of Recent Advances by O. Massarweh, A. Abushaikha; Hamad bin Khalifa University
Synthesis of ZnO Nanoparticles for Oil–Water Interfacial Tension Reduction in Enhanced Oil Recovery by H. Soleimani, N. Yahya, Universiti Teknologi Petronas; M. Baig, et. al.

Piergiuseppe Fiore is a petroleum engineer at Eni. He is part of the reservoir management and production data analysis unit, working on injectivity issues, improved oil recovery, and carbon capture and storage. He has also specialized in CFD simulations, publishing several articles. Fiore is an active member of SPE and is part of the YP Board of the SPE Italian Section. He holds an MSc degree in chemical engineering from the University of Calabria and a second-level master’s in petroleum engineering from Polytechnic of Turin.

Rami Harkouss teaches at Beirut Arab University (BAU) and is the faculty advisor of the SPE BAU chapter. Harkouss is an active participant in society. He is a member of the Lebanese Order of Engineers and a representative of BAU in Gas and Oil Processing, a European Lebanese Cooperation project. He is a reviewer of technical articles and has published papers on his research. Harkouss is the chairman of the NL TC 67 Committee responsible for the “materials, equipment, and offshore structures for petroleum, petrochemical, and natural gas industries” in Lebanon.
Harkouss holds a BE in drilling engineering from Petroleum University of Technology (Ahwaz-Iran), a master’s degree from École Nationale Supérieure de Chimie de Clermont-Ferrand, and a PhD in process chemical engineering from École Nationale Supérieure des Industries Chimiques, France.

