Climate change is one of the biggest challenges facing the planet today. Carbon dioxide (CO2) emissions are a major contributor to this problem. CO2 emissions cause the earth’s temperature to rise, leading to various negative impacts. Actions need to be taken to combat these impacts by reducing CO2 emissions. This is where CO2 sequestration comes in—capturing and storing CO2 in deep geological formations before it is released into the atmosphere. Therefore, the amount of greenhouse gases that are contributing to climate change can be reduced. Deep formations such as depleted oil/gas reservoirs, basaltic formations, coal seam beds, and saline aquifers provide various opportunities for us to explore and exploit.
The effectiveness of sequestering CO2 into deep reservoirs depends on the reservoir storage capacity, stability, and risk of leakage. Although each storage system has advantages, injecting CO2 into porous basalt rocks has been identified as one of the most promising techniques for safe and fast CO2 storage.
One of the emerging technologies regarding CO2 storage in geological structures explored on a small scale in Iceland is CO2 storage in basalt. The idea behind this process is to inject water with dissolved CO2 into reactive rocks, such as basalt, resulting in the mineralization of CO2 for permanent storage.
Mineral trapping of CO2 is one of the secondary mechanisms that can enhance CO2 storage. Three secondary trapping mechanisms are residual trapping through capillary pressure, solubility trapping through the dissolution of CO2 into aqueous pore fluid, and mineral trapping by a chemical reaction between rock, CO2, and pore fluid. Fig. 1 shows possible trapping mechanisms and their storage security. The contribution of each trapping mechanism depends on the geology of the reservoir. Due to the absence of reactive minerals, sedimentary rocks, dominant in quartz, are less likely to trap CO2 through mineralization. On the other hand, basalt’s formation can mineralize almost 90% of the injected CO2 in a few months to decades (Fig. 1, right).

To mineralize CO2 in geo-media, it needs to be bubbled into the water to dissolve it and create an acidic solution. This acidic fluid will allow the cation-bearing silicate minerals to be dissolute. The dissolution of minerals neutralizes the acidic fluid, triggering the carbonate precipitation reaction. The reaction’s pathway is shown in Fig. 2.
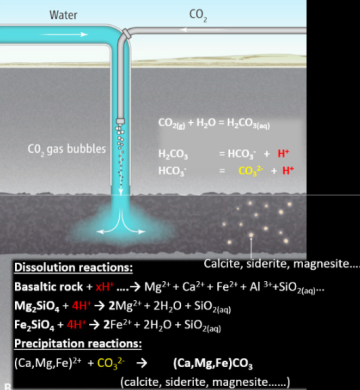
Many factors affect how efficient carbon mineralization is. Three main factors can be considered for basaltic rocks: the reservoir storage capacity, injectivity, and reactivity of the reservoir rock. The first factor is the porosity of the rock, which affects the surface area available for the CO2 to interact and be stored. Another vital factor for efficient geological storage of CO2 is the permeability of the formation, which greatly affects the injection rates. Higher permeability allows for high injection rates. The third factor is the reactivity of the rock. These rocks comprise minerals that include divalent metal cations. If the cations are unstable, the reaction will occur faster because the cations are far from the equilibrium constant. This results in faster dissolution rates in the more reactive rock and rapid mineralization.
An important factor to account for when choosing the right reservoir is the composition of the rock. Basalt is an igneous rock mainly composed of plagioclase and pyroxene minerals. These minerals are abundant in metallic cations, allowing CO2 to react chemically and form carbonates.
Through the analysis of an x-ray diffraction test, we can differentiate basalt rocks from other igneous rocks. Consequently, we will be better equipped to choose the appropriate basaltic formation for permanent CO2 storage.
Challenges
Even though the idea of storing CO2 permanently in basalt formations seems like an ideal solution, there are a few challenges and concerns to consider:
- Large water consumption is required for the mineralization process.
- In situ carbon mineralization cannot be monitored directly due to the layered basalt formations causing challenges with imaging subsurface (Raza, et al. 2022) Glatz, Gholami, Mahmoud, & Alafnan, 2022).
- Basalt formations are inherently heterogeneous because of their original depositional environment. Hence, choosing the appropriate location/formation to mineralize the CO2 always comes with a risk.
CO2 sequestration in deep geological formations is an innovative approach to addressing the challenge of climate change. Recently, CO2 sequestration in basalt received attention due to its ability to mineralize CO2 for safer and faster storage. Basalt identification is critical to ensure that suitable sites are chosen for this process. Even though there are challenges to overcome in this method, such as water consumption, the potential benefits are significant. As research in this area continues, it is clear that CO2 sequestration in basalt will play an important role in creating a more sustainable future.
For Further Reading
National Academies of Sciences (2019). Negative Emissions Technologies and Reliable Sequestration: A Research Agenda. Washington, DC: The National Academies Press.
Gíslason, S.R., Sigurdardóttir, H., Aradóttir, E.S., and Oelkers, E.H. (2018). A Brief History of CarbFix: Challenges and Victories of the Project’s Pilot Phase. Energy Procedia.
Raza, A., Glatz, G., Gholami, R., Mahmoud, M., and Alafnan, S. (2022). Carbon Mineralization and Geological Storage of CO2 in Basalt: Mechanisms and Technical Challenges. Earth-Science Review.
Voight, M. (2022). New Technology Accelerates Mineral Trapping of Carbon Dioxide. The Way Ahead.


