Technological advances in multiple-stage hydraulic fracturing and horizontal drilling have improved the overall profitability of oil-shale plays by enhancing matrix/wellbore connectivity. However, as the reservoir matures, primary-production mechanisms no longer drive oil to the hydraulic fractures, making the improvement of matrix/wellbore connectivity insufficient to provide economically attractive production rates. This study presents experimental results on the use of carbon dioxide (CO2) as an enhanced-oil-recovery (EOR) agent in preserved, rotary sidewall reservoir core samples with negligible permeability.
Introduction
CO2 is a powerful agent for EOR. It reaches miscibility at lower reservoir pressure compared with nitrogen and hydrocarbon gases, swells the oil, reduces oil viscosity, and reaches supercritical state at the pressure and temperature of most oil reservoirs, resulting in oil-like density that reduces override effects. Moreover, CO2 has been reported to be successful during field applications under unfavorable conditions such as those found with heavy-oil reservoirs and oil-wet naturally fractured reservoirs, where waterflooding is largely unsuccessful. The purpose of this investigation is to evaluate CO2 EOR in unconventional liquid reservoirs with lower permeability than that reported previously. We used preserved sidewall shale cores saturated with oil and with permeability in the nanodarcy range, preventing us from performing CO2 flooding as conventionally conceived because CO2 could not be injected directly into the matrix. We developed a technique to pack the sidewall core samples into the core holder, using glass beads to simulate the presence of a hydraulic fracture. With this approach, we did not have to cut the cores and we did not alter the rock properties and the original fluid saturation. The core was soaked in CO2 for several days, and production was allowed in intervals. Changes in saturations were tracked with X-ray computed tomography (CT). Analysis of the images revealed that CO2 was able to penetrate the cores, resulting in an oil recovery estimated in the range of 18 to 55% of original oil in place (OOIP).
This paper discusses the results on the basis of viscous-displacement, diffusion, and solubilization mechanisms. Additionally, the paper draws a research path using numerical simulation and laboratory experiments to evaluate the potential of these mechanisms to determine if they can support an economical continuous-CO2-injection process in reservoirs where conventional flooding cannot be performed because of adverse rock properties, and it compares that scenario on the basis of recovery and economics with a huff ’n’ puff CO2 injection.
Equipment and Procedures
Two experiments were conducted in this investigation. The temperature was 150°F for both experiments. The pressure was 3,000 psi in the first experiment and 1,600 psi in the second. Two preserved sidewall shale cores saturated with oil were used in each experiment (Fig. 1). The petrophysical properties of the rock are unknown, but rock permeability is so low that CO2 injection through the matrix was not possible. Therefore, conventional procedures to clean the cores, measure their pore volume, and resaturate them with produced oil were dismissed, a situation that prevents us from presenting balances of injected and produced fluids and accounting properly for recovery. Table 1 presents the conditions of the experiments and the dimensions of the cores used.

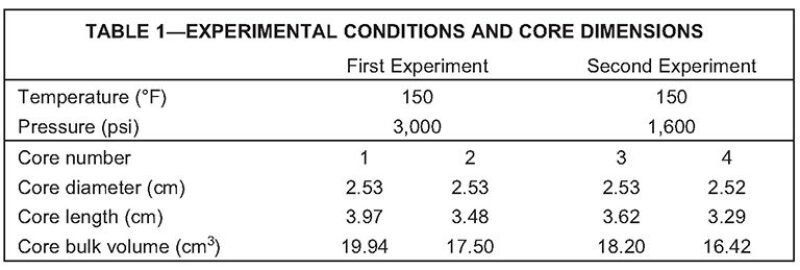
The presence of a hydraulic fracture was simulated by surrounding the cores with high-permeability media created from glass beads. This approach was selected for several reasons. The high-permeability media ensured that pure CO2 at high pressure was in contact with the shale rock at all times, which almost eliminated compositional effects; the CO2 volume in the fracture was several orders of magnitude higher than the pore volume of the core samples. This approach was chosen over cutting the core in half in order to avoid altering the core during the cutting process. The cutting element alters the rock properties by polishing the rock surface and reducing permeability. Also, the fluid used during the cutting process can imbibe into the matrix, causing important alterations because of the small pore volume. The approach selected in this investigation correctly represented the physics of a hydraulic fracture.
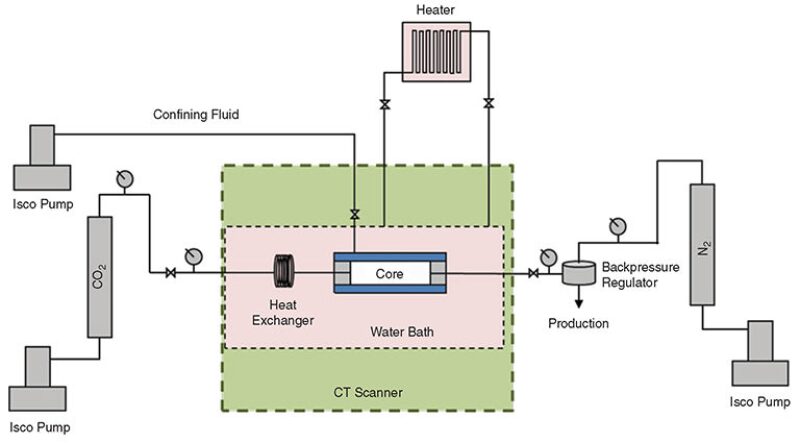
Two clean Berea sandstone samples acted as filters at either end of the setup to prevent the glass beads from entering the production tubing. Polytetrafluoroethylene shrink tubing confined the outside of the core setup. The core assembly was mounted in a Hassler-type core holder. The core holder was placed in a water bath that was heated to reservoir temperature; an even temperature was kept by circulating heated water continuously.
The whole assembly was mounted inside a medical CT scanner to monitor changes in density. Distilled water was used as confinement fluid, and the pressure was kept 250 psi above the reservoir pressure. A dome-loaded backpressure regulator kept the reservoir pressure at 3,000 psi during the first experiment and at 1,600 psi during the second experiment. The system was pressurized to 100 psi below the backpressure with CO2, which was injected through an accumulator. When the desired pressure and temperature were reached, the system was isolated. Twice a day, the pressure in the system was increased to above the backpressure, resulting in production of CO2 and any oil from the core samples. After approximately 1 hour of production, the pressure was again stabilized to a level immediately below the backpressure. Thus, production was allowed in stages. This approach was chosen because of the high volume of CO2 compared with the pore volume of the core samples. The CO2 saturation in the high-permeability media is therefore assumed to be close to unity throughout the experiments. CT scans were taken at approximately 6-hour intervals to observe any changes in the density of the system. Fig. 2 shows a schematic of the displacement equipment.
Results
Oil Recovery and Core Mass Increment. In each of the experiments, approximately 0.4 cm3 of oil was recovered. As discussed previously, porosity of the cores is unknown, and therefore OOIP and recovery factor cannot be calculated. To estimate the performance of CO2 for EOR in these cores, we set up a number of scenarios. Porosity was given a range from 0.3 to 0.6% on the basis of published data from the field from which the cores were taken. No evidence of water saturation was found during the course of our experiments because no water production was observed. However, the presence of water in the core was not discarded because the mechanism responsible for the oil production in these experiments was not suitable for water production. Therefore, a range for initial water saturation from 0 to 30% was considered.
Recovery factor was high, ranging from 18 to 51% for the first experiment and from 19 to 55% for the second experiment. The oil recovered during both experiments had a lighter color than the oil produced from the source field and seemed to have low viscosity, suggesting vaporization of the hydrocarbons into the CO2 as a recovery mechanism.
The mass of the cores changed during the experiments. In the first experiment, Core 1 increased its mass by 0.07 g (from 50.48 to 50.55 g), while Core 2 increased its mass by 0.06 g (from 45.39 to 45.45 g). A decrease in mass as a result of the production of crude oil was expected. This unexpected result was attributed to the adsorption of CO2 on the organic matter contained in the cores.
CT-Number Behavior. The attenuation of X-ray intensity when passing through a material is a function of the density and composition of such material and is expressed as a CT number. For high-energy scans, density dominates over composition. The change in CT number was tracked over time during both experiments.
During the first experiment, Cores 1 and 2 showed an increase in CT number, which can be correlated with increase in density. At 3,000 psi and 150°F, CO2 has higher density than the hydrocarbon components that are likely the main constituents of the crude oil, and the increase in CT number is an indication that CO2 is able to penetrate the preserved sidewall core over time, causing an overall increase in density.
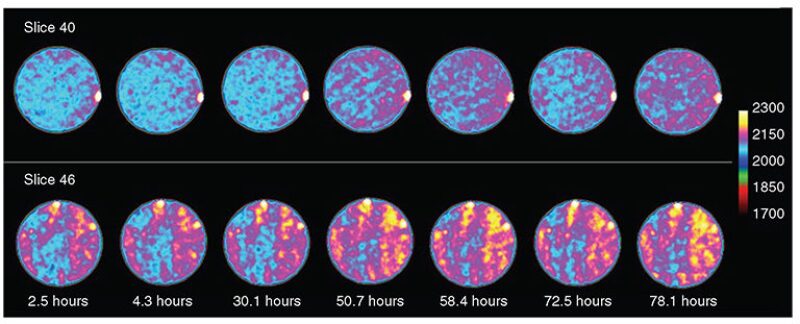
The analysis of the CT images also reveals that CO2 is making changes in the cores over time. A six-color palette was used to highlight where, in the core samples, the changes in CT numbers took place. The heterogeneities of the core sample were visible, and the color alterations occurred along the bedding planes, indicating that the penetration of CO2 into the core is influenced by rock properties and fluid-saturation patterns.
The increment in CT number is small because it follows the change in density, and the density difference between the hydrocarbons and the CO2 at 3,000 psi is not large. The authors concluded that during the soaking process, CO2 dissolves into the oil, and the lighter hydrocarbon components are vaporized into the CO2, as suggested by the appearance of the oil recovered and the CT images. The images do not show a displacement of one color by another, but a continuous change in which one color seems to fade into the next.
Fig. 3 shows CT images for Core 2 used during the first experiment. This sidewall core is more homogeneous than Core 1 and does not show bedding planes, but the increment in CT number is also evident as the images gradually change from sapphire blue to pink.
To confirm the results of the first experiment, a second experiment was performed. In the second experiment, the density difference between the CO2 and the crude oil was increased, with the crude oil being the denser fluid. The reservoir pressure was set at 1,600 psi, and the temperature was kept the same (150°F), resulting in a density of CO2 significantly lower than that seen in the previous experiment, and below the density of the selected group of hydrocarbons. The change in density caused the CT-number trend to be reversed compared with the first experiment, and a decreasing behavior was observed that suggested that the dissolution of CO2 into the oil controlled the overall density of the system. Even when the density difference was larger in the second experiment compared with the first, the changes in CT number were still small. The two reasons commented upon earlier for this phenomenon—small pore volume and adsorption of CO2 on the organic matter—are still valid in this case, and a third reason can be added. Reducing the pressure may have affected the solubilization mechanism negatively, because pressure could be lower than minimum miscibility pressure (MMP) for the second experiment. An analysis of the crude oil is not available, but given a 36°API gravity for the oil, it is very likely that MMP is lower than 3,000 psi for CO2, and therefore the first experiment was performed under miscible conditions, whereas the second could potentially be under immiscible conditions. In this case, the adsorption of CO2 on the organic matter will also tend to increase the CT numbers, cancelling out to some extent the decrease caused by the difference in density between CO2 and crude oil.
This article, written by JPT Technology Editor Chris Carpenter, contains highlights of paper SPE 169022, “Experimental Investigation of Enhanced Recovery in Unconventional Liquid Reservoirs by Use of CO2: A Look Ahead to the Future of Unconventional EOR,” by Francisco D. Tovar, SPE, Texas A&M University; Øyvind Eide, SPE, and Arne Graue, SPE, University of Bergen; and David S. Schechter, SPE, Texas A&M University, prepared for the 2014 SPE Unconventional Resources Conference, The Woodlands, Texas, USA, 1–3 April. The paper has not been peer reviewed.
