Among various CO2 sequestration methods, mineral trapping is recognized for its superior safety and extensive CO2-storage capacity. This study presents a novel methodology for assessing the rapid mineral carbonation of CO2 through geochemical interactions with carbon-, magnesium-, and iron-rich minerals abundant in geological formations. The approach and findings of the complete paper reveal that carbon storage can be successfully implemented in a matter of hours under laboratory conditions even at atmospheric pressure, effectively bridging a significant gap in the literature where experimental investigation of mineral carbonation has not been extensively explored.
Introduction
Because carbon mineralization in reservoir rocks might take thousands of years, this type of CO2 storage has been almost neglected in the literature except for a few studies where the primary focus of the experiments was merely the investigation of the alterations in the rock petrophysical and mechanical properties because of CO2 injection.
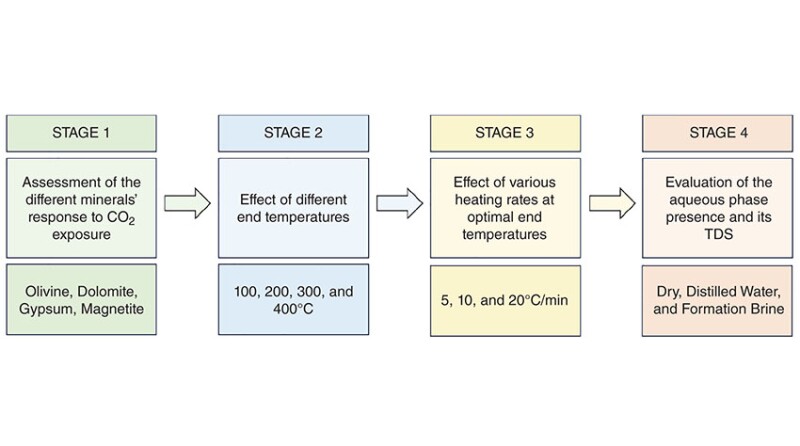
This study presents a novel approach to investigate carbon mineralization from different perspectives, including quantitative evaluation of carbon uptake by different calcium- and magnesium-rich minerals contained in the composition of most formation rocks at various experimental conditions such as temperature, heating rate, and influence of total dissolved solids (TDS) in the aqueous phase on CO2 storage on the surface of the mineral. Furthermore, apart from evaluating the carbon-uptake values, the contribution of CO2 exposure to the alteration of the surface void space of the mineral sample with and without an aqueous phase has been studied extensively.
Materials and Methods
After an extensive literature review, four magnesium-, calcium-, and iron-rich minerals were selected for CO2 exposure under various conditions: olivine, dolomite, gypsum, and magnetite.