All liquids have strong intermolecular cohesive forces between their molecules. As such, the molecules present in the bulk experience equal interaction from all directions. However, the molecules present at the interface of two immiscible liquids have a net attractive force towards the bulk of the liquid, resulting in a tension across the interface known as interfacial tension (IFT).
IFT results in the separation of two different liquids due to the cohesive forces between molecules of each liquid dominating the adhesive forces between the molecules from the other liquid. The interface thus created is a region where the molecules of either liquid are energetically less desirable to stay at.
Consequently, phases tend to minimize their interface area. As a result, increasing the interfacial area requires the work or energy input by applying any external force (e.g., shear). The lower the interfacial tension, the easier to increase the interfacial area. For oil and water systems, lowering the interfacial tension between oil and water makes it easier to emulsify the water droplet in oil and vice versa.
How is interfacial tension measured?
IFT is measured using the force measurement method (e.g., Wilhelmy plate, Du Noüy ring) where the force acting on a vertically immersed plate or ring is measured to detach it from the interface between two immiscible fluids or drop shape analysis method (e.g., pendant drop, sessile drop, growing drop, pulsating drop) or through pressure measurement (e.g., maximum bubble pressure and oscillating jet). Microfluidic devices have recently been introduced to measure IFT under different flow conditions.
Typical values of interfacial tension between crude oil and water
The typical interfacial tension values between crude oil and water range from the low 20s to high 30s mN/m and depend upon the physicochemical factors such as temperature, pressure, content of interfacially active species in the crude oil, salinity, and pH. Introducing an external surfactant can significantly lower the interfacial tension values between oil and water, sometimes to ultralow values in the 10-3 mN/m range, as done during surfactant-enhanced oil recovery programs.
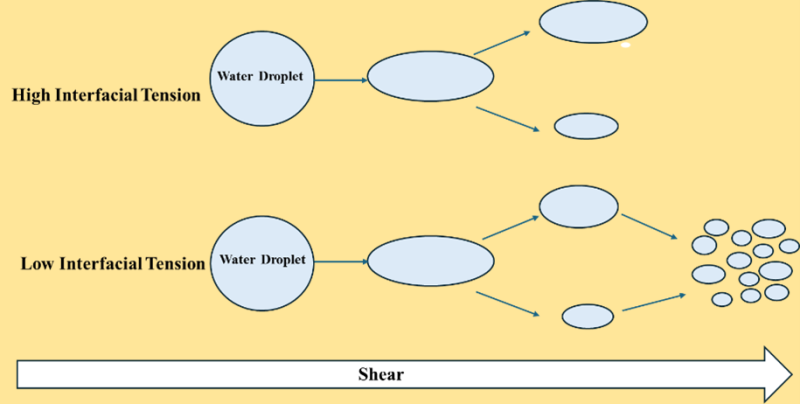
Low interfacial tension aids the emulsification of water droplets in crude oil.
The dimensionless number that governs the droplet breakup is the Capillary number or Weber number, depending on whether viscous effects or inertial effects dominate the flow or if the flow is laminar or turbulent.
- Ca = μGR/γ; the ratio of viscous stresses to interfacial stresses if viscous effects dominate the flow.
- We = ρU2R/γ; the ratio of characteristic inertial stresses in the flow to the interfacial stresses.
At a critical value of the Weber or Capillary number, a droplet in an emulsion will break up as the hydrodynamic stresses (Fig. 1) acting on the droplet overcome the interfacial tension forces holding the drop together, leading to its breakup. The is Capillary or Weber number scales as 1/γ with interfacial tension, suggesting that lowering the interfacial tension between water and oil facilitates easier breakup of a larger droplet or enhanced emulsification of one liquid into another.
During the production of oil from the reservoir, the produced fluids (water and crude) experience enough shearing that the critical drop breakup dimensionless number is easily achieved given the low interfacial tension between oil and water, which is further aided if there are any interfacially active production chemicals present during the transport.
Once the water droplets are emulsified, the interfacially active species present in crude aggregate at the interface give it a viscoelastic character, stabilizing the water in crude oil emulsions. This stability (Fig. 2) increases as the interfacially species concentration increases (e.g., more stable emulsion for higher asphaltene crudes).
Overall, low interfacial tension helps emulsify water in crude oil.
Low interfacial tension aids the demulsification of water in crude oil emulsions.
When droplets are forced together by various forces (hydrodynamic, gravitational, Brownian, etc.), the intervening liquid film drains, increasing pressure within the film. This pressure increase is related to the decreasing radius of curvature (proportional to the square root of film thickness for spherical droplets). As the film thins, it can deform into a dimpled shape as the film pressure approaches the Laplace pressure. The Laplace pressure (ΔP = 2γ/r), the pressure difference across a curved interface, resists this drainage, attempting to maintain droplet curvature and hindering coalescence.
Eventually, the film thins to a critical point where non-hydrodynamic (disjoining) forces dominate. Once a stable film forms, hole nucleation theory can estimate its rupture time.
The activation energy (Ea) required for hole formation and expansion between droplets depends on film thickness, interfacial tension (IFT), interfacial rigidity, and disjoining pressures FT and rigidity can be estimated using the HLD-NAC framework, depending on surfactant type, medium chemistry, and temperature.
Near the phase inversion point, IFT can be low, leading to low activation energies. Consistent with theory, the activation energy for drainage and coalescence increases linearly with increasing IFT.
This is one of the action mechanisms of demulsifiers that are used in the industry for crude oil emulsion mitigation, i.e., they work by reducing the interfacial tension between the oil and water phases, which allows easier coalescence of water droplets in the continuous medium (demulsifiers also alter the interfacial mechanical properties).
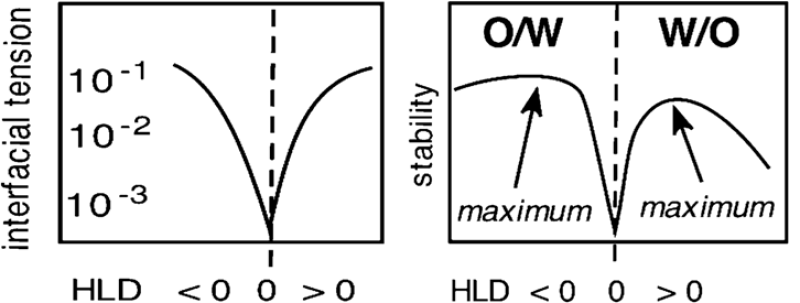
From a formulation point of view, the minimum interfacial tension corresponds to a point of balanced formulation condition corresponding to a hydrophilic-lipophilic difference (HLD) of 0.
HLD quantifies the difference in chemical potential of interfacial species between oil and water phases and considers the chemical character of the oil and water phases, the salinity of the system, and temperature. Apart from IFT, other interfacial dilational rheological properties also pass through a minimum at HLD of 0.
Practical implications for crude oil production
Crude oil is produced as an emulsion where the formation water or injected water emulsifies in the crude oil as early as within the porous media. However, the droplet size distribution is reduced further when the emulsion experiences further shearing. Therefore, injection of interfacially active species upstream that could potentially reduce the interfacial tension to facilitate further emulsification should be avoided in the field, especially at the concentration where the HLD of the system may shift to maximum stability of the emulsions.
Once the produced water in crude oil emulsions is ready for transportation through pipelines, it is crucial to do a sensitivity study on further emulsification of water droplets due to the flow field at the existing interfacial tensions.
Finally, it is critical to understand how adding demulsifiers affects the interfacial tension between oil and water in oil-water separation vessels. Demulsifiers counteract the effects of natural emulsifiers present in the crude that stabilize the water droplets in the oil.
While the overall mechanism involves modifying interfacial properties and flocculation of droplets, the IFT reduction by injecting the demulsifier should be near the minimum interfacial tension achievable for the demulsifier-oil-water system, i.e., around HLD approximately 0. Another important factor when considering the low interfacial tension is the presence of excessive shearing near HLD = 0, as the oil-water mixture can be emulsified with minimal energy due to the ultralow interfacial tension values.
Therefore, the demulsifier selected for a given oil and aqueous phase combination must be chosen such that the HLD of nearly 0 to ensure the lowest interfacial tensions that allow drop coalescence.
In conclusion, the interfacial tension is an important parameter that dictates the behavior of crude oil-water system where low interfacial tensions aids both emulsification as well as demulsification of water in crude oil.
Lowering the interfacial tension reduces the extent of shearing required for water droplet breakup which potentially increases the emulsion tightness and should be avoided during the transport of crude from wellhead to the separation plant. However, once the produced emulsion reaches the oil-water separation plant, lowering the interfacial tension should be a priority for effective oil-water separation.
Finally, the emulsion should not experience intense shearing after the injection of the demulsifier, as the lower interfacial tension values can stabilize the emulsions severely if the oil-water-demulsifier formulation is away from the optimum formulation condition.
For Further Reading
Measurement of interfacial Tension in Fluid-Fluid Systems by J. Drelich, C. Fang, and C. White. Encyclopedia of Surface and Colloid Science (2002).
Emulsion Characterization via Microfluidic Devices: A Review on Interfacial Tension and Stability To Coalescence by TM Ho, A Razzaghi, A Ramachandran, KS Mikkonen. Advances in Colloid and Interface Science (2021).
Breaking of Water-In-Crude Oil Emulsions. Part 9. New Interfacial Rheology Characteristics Measured Using a Spinning Drop Rheometer at Optimum Formulation by M. Forgiarini, D. Langevin, J.S. Salager, R. MarquezAna Energy Fuels (2019).
Emulsion Stabilization, Breaking, and Inversion Depends Upon Formulation: Advantage or Inconvenience in Flow Assurance by J.l. Salager, A.M. Forgiarini. Energy Fuels (2012).


