If a liquid takes its container's shape, then what elements of the container go with the liquid when pumped out? How can these elements be extracted from the liquid and refined into valuable products that contribute to society in various ways?
These are just some of the many questions that researchers at Texas A&M University are attempting to answer as the world works to reduce its dependence on nonrenewable energy sources, secure supply chains for critical minerals, and find value where others see none.
Take produced water, for example.
Billions of barrels of water are produced daily from oil and gas reservoirs around the globe, creating a management challenge of gargantuan proportion as the industry seeks to reuse or dispose of it.
However, Hamidreza Samouei, a research assistant professor in the university’s Harold Vance Department of Petroleum Engineering, takes a different view of the briny brew, calling it an overlooked liquid gold.
“It is a millennia-old cocktail of dissolved minerals interacting with the subsurface for eons. This water has accumulated minerals to the point where the total dissolved salts can be ten times that of seawater,” he said.
“Virtually every element on the periodic table, albeit in varying quantities, can potentially be sourced from this water. Yet, due to a combination of technological constraints and limited interest, this mineral-rich water is often reinjected into reservoirs untreated, a veritable treasure trove discarded daily,” added Samouei.
On the other hand, CO2 emissions are also a challenge the world is grappling with managing and mitigating. But what if there was a way to combine the two waste products—produced water and CO2—and transform them into value-added products?
Brine Refining
Samouei and his research team have developed a process using water desalination to merge the two, resulting in mineral extraction from the briny water and capturing the CO2 by mineralization.
Water desalination holds the key to sustainable access to fresh water, but there are two critical challenges to establishing new desalination facilities, according to Samouei. One is the environmental impact of the produced brine, and the other is the CO2 emissions from the fossil-fuel-based energy sources for the facilities (Fig. 1).
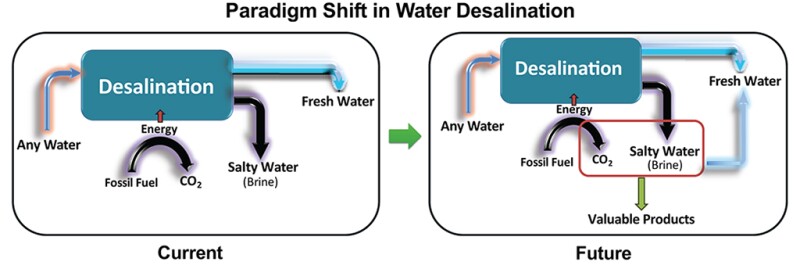
“Brine mining is a concept that has the potential to address the environmental concerns tied to brine disposal,” he said, adding, "by harnessing methods that allow for the extraction of valuable minerals from brine to unlock economic opportunities through the creation of marketable products.”
He said society can overcome economic, environmental, and social barriers through a convergence approach that addresses both brine disposal and CO2 emissions and transforms them into value-added products, including critical minerals.
“This holistic strategy has the power to enhance sustainability, circular economy, and inclusivity, ultimately ensuring an ample supply of fresh water accessible to society as a whole,” he said. This approach offers three advantages simultaneously: monetizing brine, CO2 capture, and making fresh water.
Brines generally have four major cations: sodium, potassium, calcium, and magnesium.
The brine refining process (Fig. 2) demonstrates how when CO2 is introduced to each element, the chemical reaction results in products used in various commercial and industrial applications, including calcium carbonate (CaCO3), magnesium carbonate (MgCO3), sodium bicarbonate (NaHCO3), and potassium bicarbonate (KHCO3). These products are used as building materials, road aggregates, fertilizers, glassmaking, and many more applications.
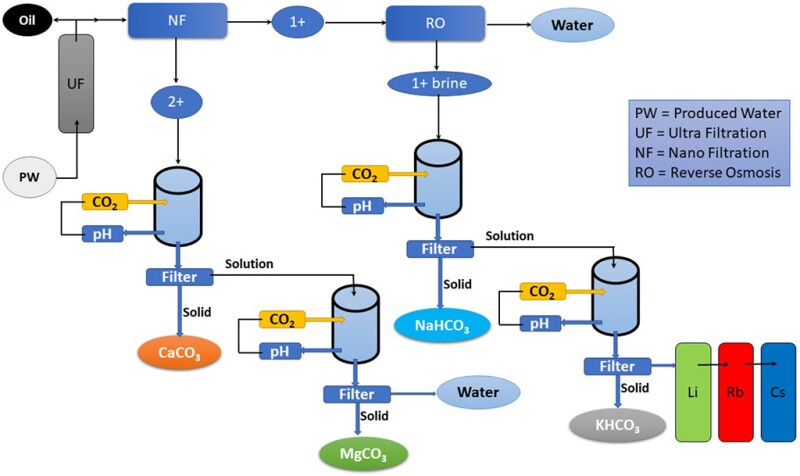
Further filtration of potassium bicarbonate refined from brine water could result in lithium (Li), rubidium (Rb), and cesium (Cs) in more concentrated form, all important critical minerals in electric vehicle batteries and photoelectric cells. Eventually, after the precipitation of salts, fresh water will be produced.
Additionally, each reaction sequesters CO2 in what Samouei sees as one of the “most considerable capacities and opportunities to trap CO2 in the world.”
For example, for 1.0 kg of sodium chloride (NaCl), 0.75 kg of CO2 is sequestered (Fig. 3).
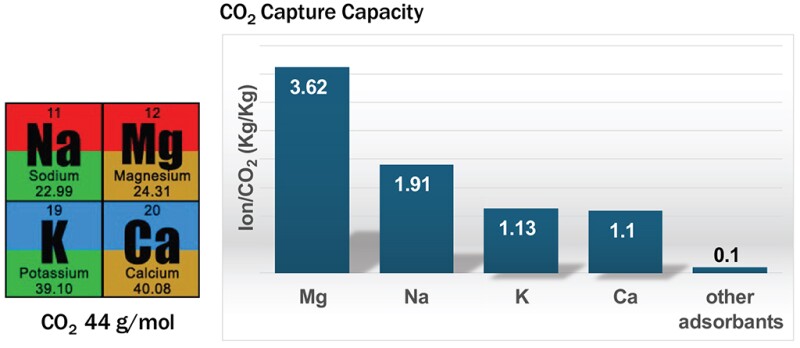
“Moreover, inexpensive NaCl ($70 per ton, 40% Na) will be converted to more expensive ($300 per ton, 20% Na) with significantly broader applications and market size,” he said.
“Sodium bicarbonate production is more than 40 million tonnes, which is about 6 kg per year for each person on Earth. This process can be 95% carbon negative by considering the energy for the seawater desalination comes from fossil fuels, and for 1 m3 of water, 3.4 kW per hour of electricity are consumed, and 1.31 kg of CO2 is produced.”
Paradigm Shift
Samuei said that there’s tremendous interest in mining the deep ocean floor for ancient, mineral-rich nodules, and some are looking up to celestial bodies like asteroids for critical minerals. Harnessing the power of produced water and CO2 presents a more realistic and sustainable solution to mineral scarcity.
“Sometimes the answers lie closer to home,” he said.


