As the daily oil output of the Bakken and Eagle Ford formations rose toward 1 million B/D, researchers were seeking a way to push the ultimate recoveries in these formations, where producing 6% of the oil in the ground is now considered good.
One line of attack on the problem is using carbon dioxide (CO2) to get more oil from tight formations where rapid production declines are the norm.
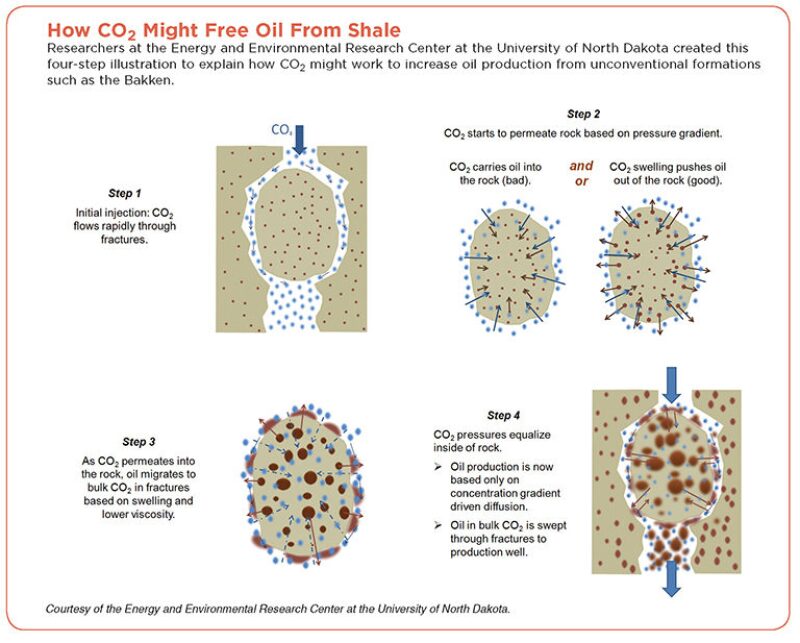
In laboratories at the Energy and Environmental Research Center (EERC) at the University of North Dakota and at Texas A&M University, experiments have shown that carbon dioxide circulated around a small sample of source rock can remove a significant amount of oil. Now, the scientists are trying to understand how it works and if those lab results can be applied in the real world.
“It is incredible what CO2 can do,” said John Harju, associate director for research at the EERC, while describing the center’s research program at the annual CO2 Flooding Conference in Midland, Texas.
“The really big prize is (overcoming) the innately low recovery rate in these shale plays,” said David Schechter, an associate professor of petroleum engineering at Texas A&M University who is turning his expertise in conventional enhanced oil recovery to unconventional reservoirs.
While laboratory results normally show much higher recovery than field results, even a 1% improvement of recoveries in the Bakken formation could yield more than 1 billion bbl of oil, according to an EERC paper.
Previous SPE papers, based on reservoir simulation work at Montana Tech of the University of Montana and the Colorado School of Mines, concluded that significant increases in ultimate oil recovery might be possible using CO2 injections.
One of the biggest backers of the work in North Dakota has been Harold Hamm, the chief executive officer at Continental Resources, which pioneered unconventional liquids development and is the biggest acreage holder in the Bakken, Harju said.
On the basis of early tests using CO2 and similar positive results from using chemical surfactants, Texas A&M is working to recruit support from oil companies for a joint industry project called the Enhanced Oil Recovery in Unconventional Reservoirs Joint Industry Project.

Both the EERC and Texas A&M have tested samples ranging in size from Chiclets—square bits of reservoir rock about the size of the popular chewing gum—to small core samples about the size of a disposable lighter. The tests produced significant amounts of oil from samples exposed to CO2 flowing through a test chamber simulating reservoir conditions.
In a lab test, the EERC was able to recover 60 to 95% of the hydrocarbon in rock samples from the middle, upper, and lower Bakken, according to a paper on the work (SPE 167200). Researchers were surprised to find that the recovery rate at reservoir pressures and temperatures was high for all three layers after 4 days of exposure.
The middle Bakken reached that recovery rate faster than the tighter rock in the upper and lower Bakken. The result in the middle Bakken was not surprising because that rock is not as tight as the layers of source rock above and below it. Researchers reported oil recoveries ranging from 60 to 80% from the upper and lower Bakken after longer exposures to CO2.
In a test at Texas A&M, a small sample produced 0.4 cm3 of oil, suggesting a potential recovery rate of more than 18% of the oil in the sample. Schechter said testing so far suggests the CO2 is “dragged into the matrix” of the rock. The laboratory there also found that using chemical surfactants produced similar results
In conventional fields, injecting CO2 aids production in several ways: by reducing interfacial tension, which loosens the hold of the rock and oil; by lowering the viscosity of the oil; and by causing oil molecules to swell, forcing it out of pores in the rock.
CO2 is high on the list of EOR methods to try because the options are limited. Many unconventional reservoirs, including the Bakken, are oil-wet, and that attraction between the rock and the oil means waterflooding is very unlikely to succeed, said Ed Steadman, a department director at the EERC.
Researchers in North Dakota have begun working to understand how CO2 is likely to act in the Bakken formation. The expectation is that these tight reservoir rocks, where the gas must flow through constricted fracture networks, will act differently than porous conventional formations.
“Testing on Bakken rock suggests CO2’s benefits will require significant contact time,” said Steven B. Hawthorne, a senior research manager at the EERC.
Another question is: What does it take for CO2 to become miscible with oil in shale reservoirs? The conditions at which carbon dioxide is miscible with oil matter because that is the point when it is most effective at getting more oil out of a reservoir. Carbon dioxide floods are engineered to reach the minimum miscibility pressure (MMP)—the level where there is no interfacial tension between the oil and CO2.

At the CO2 conference, Hawthorne showed a video offering a view of how CO2 interacts with oil as the pressure rises to where it becomes miscible and beyond. The image of what went on inside a small tube simulating reservoir conditions in the Bakken showed a rising level of activity as pressure increased, suggesting the line between immiscible and miscible could be fuzzier than is suggested by precise calculations of the MMP.
In the Ground
All the researchers involved say more work is needed before they can say this will work in the field. “If it contacts the rock enough, under the right conditions, this could work,” Schechter said.
Two field tests using CO2 in the Elm Coulee field in Montana in 2009 and in Mountrail County in North Dakota were described by Harju as “not particularly successful.” What has been learned will be applied in two to three tests expected over the next year.
It could be a long journey. The first two CO2 EOR tests tried injecting carbon dioxide into a field, closing off the well, and then returning it to production to see if the CO2 increased the output. These “huff ’n puff” tests were not successful, which is leading researchers to consider ways to push a stream of the gas through the rock to maximize the surface area and the duration of the CO2 contact.
Researchers in North Dakota are considering injecting carbon dioxide inside the oil-rich shale layers that sandwich the middle Bakken—the layer of dolomite that has been the primary source of production there—and the Three Forks, which is also being produced.
Finding an effective CO2 EOR method would only be a first step. Applying it widely in a shale play covering half of a state and several adjoining states and provinces would require enormous amounts of carbon dioxide in an area where there is not enough CO2 to flood conventional reservoirs left from the early days of oil production.
Grand Plan
Filling those projected needs could alter the economic landscape of North Dakota. Because the state needs more power and has large undeveloped coal resources, one idea is to capture carbon dioxide from new coal-fired power plants. It could generate more power, enhance oil production, create a market for the coal, and emit little or no CO2. It would also make the state a technology proving ground.
North Dakota has estimated it would need an additional 2,500 MW of electric-generating capacity in the future, much of it to support growing oil production, Harju said. That is double the state’s current capacity, according to the US Energy Information Administration.
That plan could create demand for a less-desirable resource—lignite, which is a low-quality grade of coal that needs to be burned near to where it is mined because it is expensive to ship.
Realizing this vision will require reducing the cost of removing CO2 from the exhaust gasses vented from power plants burning coal. Another option would be producing CO2 using the natural gas associated with oil production in the Bakken, much of which is now flared. Either way, the technical risks are large, but so might be the potential returns.
For Further Reading
SPE 167209 Long Overlooked Residual Oil Zones (ROZ’s) Are Brought to the Limelight by A Harouaka, B. Trentham, University of Texas of the Permian Basin, and S. Melzer, Melzer Consulting
SPE 123176 CO2 Flooding the Elm Coulee Field by Shehbaz Shoaib, SPE, Montana Tech, and B. Todd Hoffman, SPE, DRC Consulting
SPE 168915 Geologic Characterization of a Bakken Reservoir for Potential CO2 EOR by Basak Kurtoglu, Marathon Oil Company, and James A. Sorensen, Jason Energy and Environmental Research Center, et al.
SPE 167200 Hydrocarbon Mobilization Mechanisms From Upper, Middle, and Lower Bakken Reservoir Rocks Exposed to CO2 by Steven B. Hawthorne and Charles D. Gorecki, Energy and Environmental Research Center, et al.
SPE 168774 Hydraulic Fracture Orientation for Miscible Gas Injection EOR in Unconventional Oil Reservoirs by Tao Xu and Todd Hoffman, Colorado School of Mines

