Gummy bears is one of the names for a gross mix of downhole junk that can foul a separator or clog tubing in the Woodford play in Oklahoma.
The texture is often rubbery—the only connection to the gummy candy—and the color varies depending on whether the well’s output tilts toward oil or gas.
These “unusual semi-solid” accumulations were described in a 2015 Halliburton paper as containing hydrocarbons, sand, iron, fine particles from clay, and sometimes a bit of friction reducer—polyacrylamide to be exact (SPE 173594).
The globs generally are seen at the surface after fracturing. When they are observed downhole, they have been pushed by the well’s flow into “choke points” such as “perforations, tubing anchors, gas lift valves” downhole which are noticed when production declines, the paper said.
Analysis revealed that those semi-solid amalgams contained iron, which was not in the injected fracturing fluid. While the nature of the stuff made polyacrylamide a likely ingredient, they did not find acrylamide—the ingredient combined with polymers to make friction reducer.
The paper suggested that fracturing “released materials with a strong positive charge (cations)” which were part of “a complex mixture including other solids and the acrylamide, forming a tight emulsion in semi-solid form.”
The paper offered a workable description of what was going on below. But the analysis and solutions offered did not seem to make a dent in the problems that were so bad for some operators in the Woodford that they switched from friction reducer to guar to avoid the gunk.
Guar, a natural product is not as effective as polyacrylamide at reducing friction. While it has long been used to thicken water-based fluids and allow them to deliver more proppant, laboratory testing concluded guar can hinder production.
Substituting guar “is not ideal because the residue guar can [be left] behind on a proppant pack,” said Mark Van Domelen, vice president for technology at Downhole Chemical Solutions (DCS).
The maker and supplier of fracture chemicals has been working with Ovintiv on a way to pump friction reducer without the gummy side effects. Ovintiv, formerly known as Encana, saw improved chemistry as a way to increase production from the Oklahoma acreage it acquired from Newfield Exploration.
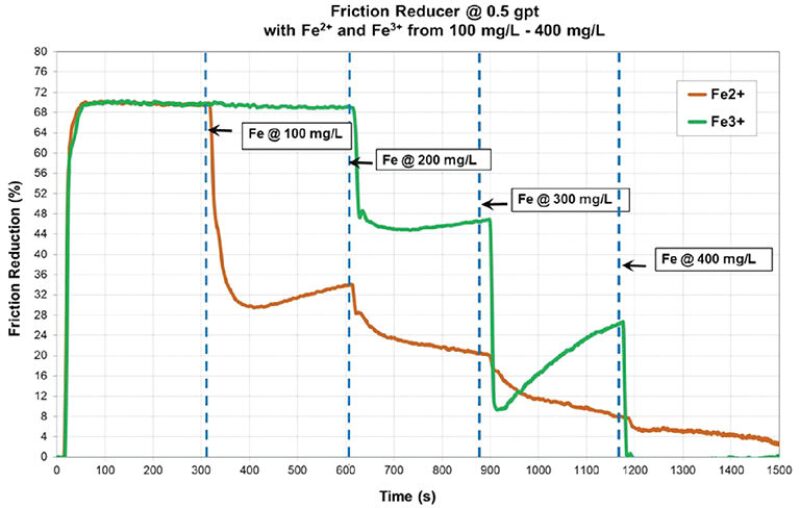
The research partners tested the widely held theory that the gunk was created by a reaction between polyacrylamide and pyrite (iron sulfide or FeS2) in fresh water and found, “When an iron source is added to the fluid a nearly instantaneous development of the accumulation material was noted,” according to their paper (URTeC 2487).
A Determined Operator
The partners identified the source of the obvious problem—the gummy bears—and some less obvious ones that could undercut the effectiveness of chemicals used for fracturing.
One of those is the widely used practice of injecting acid on a site before it is stimulated, which is known as an acid spearhead.
Ovintiv’s testing showed that as acid dissolved the rock, which is supposed to increase the odds that a fracture will develop, it released minerals likely to react with friction reducers, including magnesium and iron.
Other iron sources include metal from tubulars and the produced water used for fracturing.
DCS then tested in a flow loop how friction reducer reacts to iron and found that adding iron quickly reduced that chemical’s ability to make the water slick—which increased the energy required to pump the fluid down a long lateral (SPE 195199).
Another test showed that adding iron reduced the ability of the friction reducer to thicken the fluid, reducing its ability to effectively carry sand deep into a fracture.

Beyond the Woodford
Iron is one of the most abundant elements on earth, but for some reason gunk research has been laser-focused on Oklahoma plays, particularly in the Woodford.
“Iron is ever-present in fracturing operations,” Van Domelen said. Still, when it comes to the gummy bears, “we don’t hear much about it happening in the Permian.”
That could be the result of a natural reluctance of operators to talk about plugged wells with people who were not involved in those jobs.
Or it could be that the chemistry in Oklahoma shale plays has a unique tendency to produce the gunk, which has long been a topic of conversation.
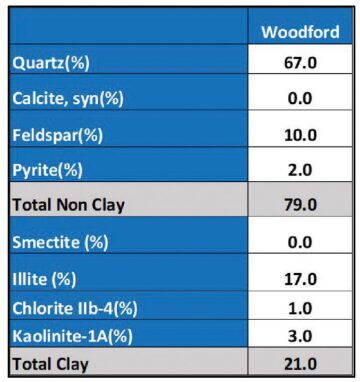
High levels of iron in the Woodford are a critical ingredient in gunk formation. But the Marcellus Shale in Pennsylvania has similarly high pyrite levels—at around 2%.
Alexandra Hakala, a research scientist for the National Energy Technology Laboratory (NETL) in Pittsburgh, Pennsylvania, never saw the gunk until she saw an image in the recent URTeC paper about the problem and how to treat it.
The NETL Onshore Unconventional Resources Technical Portfolio lead immediately began looking for a sample to see if it could offer insights into the chemical and biological interactions that create the oddities.
As for why it has not been reported in the Marcellus, Hakala said she is “not sure if operators have encountered it and just haven’t reported on it, or it all stays downhole, so nobody knows if there’s a problem.”
While shale plays often have the iron or other reactive chemicals needed to turn friction reducers into gummy bears, other factors may play a role in producing these semi-solids. She said those include brine chemistry, kerogen/organic composition, or the mix of additives in the fluid.
It does not just happen in Oklahoma. There are instances of polyacrylamide plugging wells going back to conventional well treatments done around 1990.
Two Amoco wells in Brownfield, Texas, southwest of Lubbock, were “plugged up with a very stable gunk following some of the first uses of polyacrylamide as an acid gelling system. The viscosity of the mass was similar to a partially set glue and at least one of the wells” had to be plugged and abandoned, said George King, an engineering advisor with Viking Engineering.
While the Marcellus has not offered an opportunity to study gunk, a NETL study found evidence that iron can also react with surfactants, undercutting the ability of those additives to increase oil production.
Once again, iron in the form of pyrite was in the spotlight. “Pyrite caused the most degradation—pyrite is very prevalent in shale,” said Lauren Burrows, a research fellow at NETL’s Pittsburgh laboratory, who presented a paper on the work (URTeC 561) in 2019.
The transformation meant that additives lost their ability to free oil by changing oil-attracting rock surfaces into ones that attract water. “Those chemicals are not doing their job if they are degrading in a couple hours,” she said.
The same could be said about friction reducers that lose their ability to reduce friction when exposed to iron. In both cases, though, it is hard to know what is happening a couple miles down in a well.
Downhole Reactions
The surfactant research project began with an observation: “What goes in does not come out.”
Burrows’ explanation for the change: “There is likely a transformation going on downhole changing the molecular weight” of the glycol and surfactant pumped to increase oil output.
They tested ground-up shale, sand, and pyrite by adding each to an amber flask containing a surfactant-glycol mix in an acidic solution warmed to simulate reservoir conditions. After 24 hours they had produced a glycol whose low molecular weight did not match either input. Worst of all, the reaction created a substance that could “potentially inhibit” oil production rather than promote it.
The barrage of questions from the audience after the presentation of URTeC 2487 showed how many other ways those working with oilfield chemicals may interpret what is happening in a well.
One of the questioners pointed out there are “a lot of other materials in frac fluid,” and asked, “Could it [pyrite] cleave to all the others?”
Concerns about frac fluids reacting to reservoir fluids have been around since the early days of shale-gas development in the Marcellus where the lack of produced-water-disposal wells forced producers to reuse produced water. Those running completions soon learned that concentrations of salt (NaCl) reduced the effectiveness of fracturing chemicals, which led to reformulated chemicals.
There were also debates about whether it was worth the cost to remove metals that might cause trouble, such as barium that can cause scale development.
Still, experts who have studied problems blamed on friction reducers complain that these additives are regularly evaluated by the industry based on tests in tap water, where they generally perform as promised.
Creating a realistic test for a widely used fracturing additive will require meeting a huge challenge: Describing the chemistry of a typical shale play.
These formations are far more complicated and variable than the relatively consistent, permeable rock in conventional reservoirs.
“Shale is a unique challenge. It tends to have more reducing components” than conventional plays, Hakala said. More minerals are found in shales, which are the organic-rich source of hydrocarbons made from organisms that lived millions of years ago. Over time oil and gas migrated to more porous and permeable sandstones and carbonates.
Previously, the challenges presented by source rocks could be ignored by the industry when there was plenty of oil to find in those conventional reservoirs.
Hakala has an unusual perspective on the chemistry of shale because her graduate research focused on how different forms of iron, such as pyrite, accumulate and react in wetlands. Microscopic images of iron compounds from the Woodford looked familiar to her.
Engineers doing completions have focused on using hydraulic force more effectively to open up productive, propped pathways in ultratight rock rather than studying the chemical reactions happening in those wells.
Based on his experience teaching about fracturing, King said engineers need to learn more about chemistry. When asked about problems associated with friction reducers, he was blunt: “Damn friction reducers and iron! Engineers do not understand chemistry!”
Many Expressions
Even those who do understand chemistry and have an advanced degree to prove it have a lot of work to do before they can create a tool to predict how injected chemicals will react in a shale well.
NETL is working on creating the inputs needed for such a system. Hakala said the modeling will depend on laboratory results measuring potential interactions in the ground. These “expressions” describe the likely outcomes when mixing various organics, chemicals, and minerals at specific temperatures and pH levels. This long-term project is expected to require another 18 months of work.
Meanwhile, Van Domelen’s presentation at URTeC offers steps to limit the troublesome reactions between friction reducers and iron based on available tools that Ovintiv is using.
- Use relatively fresh water when possible in iron-rich reservoirs because the minerals in produced water can introduce reactive components to the friction reducer from the moment they are mixed.
- Acid spearheads should be avoided, if possible, because of the detrimental substances they may cause to be released. If they must be done, a spacer stage that does not include friction reducer should be pumped to flush the acid.
- Use a clay-control additive to reduce the production of fines, which can help stabilize the polymer accumulation (gummy bears).
- Steps should also be taken to ensure the polyacrylamide breaks down after use. Use a polyacrylamide with a low to moderate molecular weight because it breaks down more cleanly than those with heavier molecular structures. The paper said, “It is reasonable to believe” higher-weight molecules would be more likely to develop polymer accumulations if exposed to pyrite, but “further work in this area is ongoing.”
- A high dose of oxidizing breaker is recommended because more is needed in an iron-rich environment to break down the friction reducer. Breaker also “very likely helps to reduce the formation of viscous accumulations” and breaks them down if they have formed.
An alternative has come on the market: friction reducers formulated to be a cation such as reactive iron, calcium, or sodium. A paper by BJ Services from 2019 URTeC said its cationic friction reducer performed well in a field test for an unnamed operator with the lower-than-expected pumping pressure indicating it remained effective in produced water with high levels of salt and calcium. Their flow-loop testing did not show that iron (Fe3) had an effect on cationic or anionic friction reducers (URTeC 129).
But so far, no field results are available from operators.
The Payoff
A skeptical engineer may well react to all this by noting that a seller of fracturing chemicals has concluded that the solution to gummy bears requires buying more chemicals plus testing and analysis services to adjust the chemicals used to alter well conditions.
And then ask what’s the payoff for all that.
Ovintiv offered an answer in the form of a nine-well test, comparing production from four wells completed this year using the techniques developed to limit the impact of iron on the friction reducer, among other changes.
In the older wells “problems were sometimes experienced related to polymer accumulations discovered in the tubulars and the separators after fracturing using polyacrylamide,” the paper said.
In the four new wells fractured early this year, the paper said no polymer accumulations were observed during or after fracturing.
Production results were adjusted for some significant differences in the wells such as the completions and choking procedures. The new wells all have two-mile-long laterals—some of the older ones are half that—and the new ones have more frac stages and proppant placed.
The paper said the adjusted 60-day production average was 750 B/D, compared to 335 B/D for the older wells.
“While a portion of the improved well production in the Pad B (2020) wells can be attributed to slightly higher proppant volumes placed (7%), the enhanced well deliverability results are encouraging,” the paper said.
It reported that changes in the fracture fluid “yielded much better well deliverability with no fluid incompatibility” issues.
An outsider can only guess how much of that production jump is a product of fluid-chemistry changes. One positive sign is there are people in the Woodford interested in finding out for themselves.
“Other operators are interested and wanting to talk to us about using it,” Van Domelen said.


